Our Mission
Puma Biotechnology is a biopharmaceutical company dedicated to the acquisition, development, and commercialization of novel therapeutics for the treatment of cancer.

Clinical Trials
ALISCA™: ALISertib in CAncer
What to Expect in a Cancer Clinical Trial
If you or a loved one has cancer, you might hear the words clinical trial. But what does that really mean? And what happens if you decide to join one?
Let’s take a look at how clinical trials generally work.
What Is a Clinical Trial?
According to the National Cancer Institute’s (NCI) online Dictionary of Cancer Terms, a clinical trial is defined as a “type of research study that tests how well new medical approaches work in people.”1
Doctors look to clinical trials to learn more about cancer and how to treat it. People join clinical trials to help test potential new treatments, like new cancer drugs or ways to reduce side effects. Clinical trials help doctors find out what works—and what doesn't.
If you and your doctor decide that a clinical trial may be a good option for you, there are several milestones it may be helpful to know about.
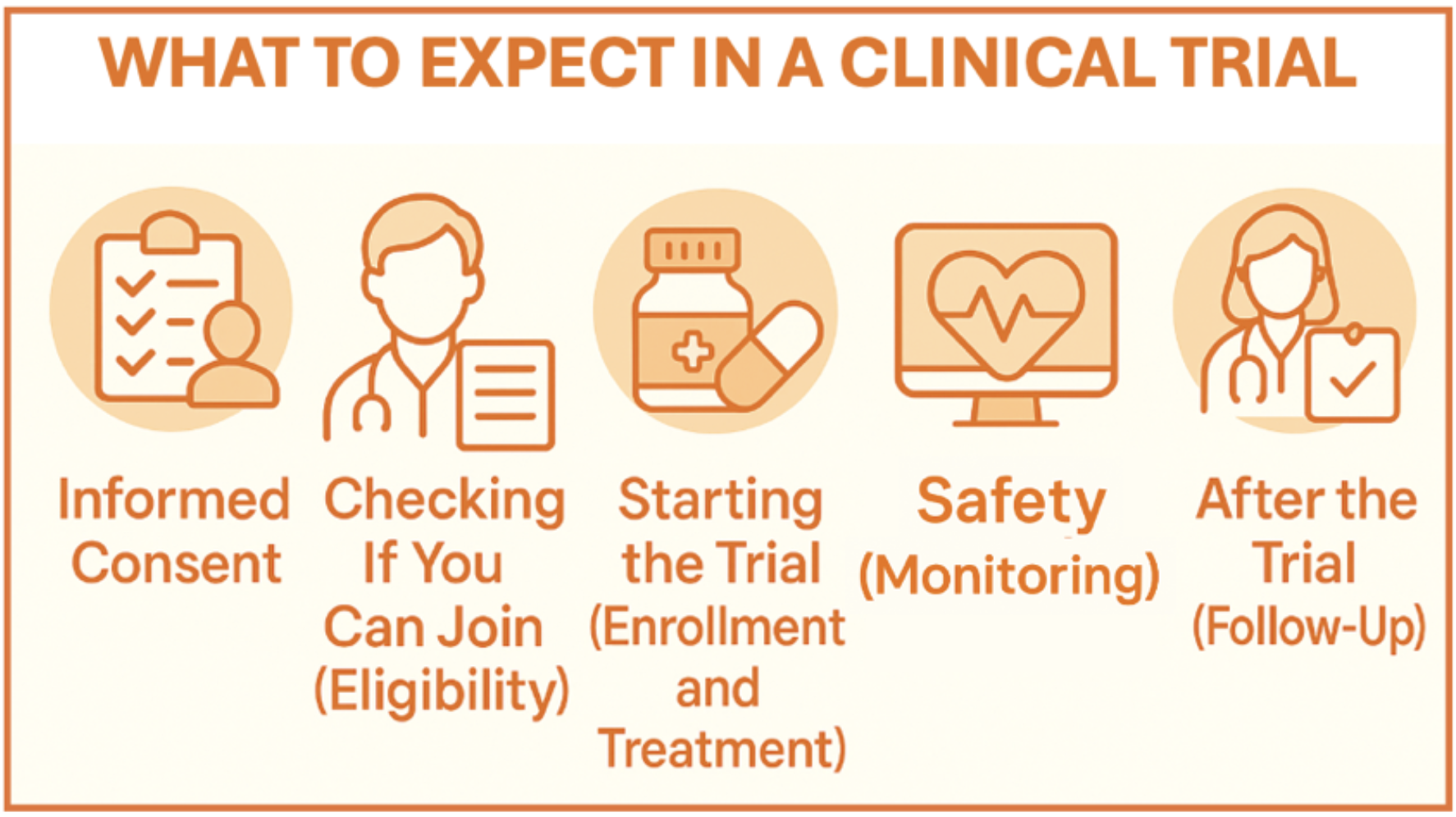
Informed Consent
During the informed consent process, the research team will:
- Explain what the trial is about
- Describe the treatment and what will happen
- Talk about possible risks and benefits
- Answer your questions2
You’ll get all of this in writing too. If you decide to join, you’ll sign a form saying you understand and agree. But even after signing, it’s important to remember, you can leave the trial at any time.2 Your participation is always voluntary.
Checking If You Can Join (Eligibility Criteria)
Every clinical trial has eligibility criteria. Eligibility criteria is a list of basic information that could include:
- The type or stage of cancer you have
- Being in a certain age range
- Treatments you’ve had before
- Your overall health and medical history3
After you complete the informed consent form, you’ll go through a screening process. This may include questions, blood tests, scans, or a physical exam which help determine if you meet the eligibility criteria for the trial.4 This is all part of making trials as safe as possible and deciding if it is a good treatment option for you.3
Starting the Trial (Enrollment and Treatment)
Once you begin the clinical trial, you might:
- Complete appointments for things like scans and blood tests
- Receive a new medication
- Try a new type of care to manage symptoms5
You’ll have regular visits with the clinical trial team.4 They’ll check your health and watch for side effects.4 They might also ask questions about how you’re feeling.4
Safety (Monitoring)
Patient safety is a priority. During a clinical trial, the clinical site team will typically:
- Watch for side effects
- Adjust the plan if needed6
If you have any problems or want to stop, you can speak up at any time. The team is there to help you.
After the Trial (Follow-Up)
When the trial ends—whether you finish it or choose to leave early— you’ll have a final appointment with the study team.4 As well, you’ll continue to be seen by your regular medical team.7
Final Thoughts
Joining a clinical trial is a big decision. It’s okay to have questions or feel unsure. You can click here to download Questions to Ask Your Oncologist – a document we created to help you talk to your doctor.
-
1
National Cancer Institute, “Dictionary of Cancer Terms,” May 16, 2024, https://www.cancer.gov/publications/dictionaries/cancer-terms/def/clinical-trial. Accessed August 28, 2025.
-
2
Shah P, Thornton I, Kopitnik NL, et al. Informed Consent. [Updated 2024 Nov 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430827/. Accessed August 28, 2025.
-
3
National Cancer Institute, “Steps to Find a Clinical Trial,” May 16, 2024, https://www.cancer.gov/research/participate/clinical-trials-search/steps. Accessed August 28, 2025.
-
4
Clinical Leader, “Site Visits 101: Visits With Participants, From Prescreening To Completion,” https://www.clinicalleader.com/doc/site-visits-visits-with-participants-from-prescreening-to-completion-0001#:~:text=End%2DOf%2DTreatment%20Visit,an%20organized%20and%20compliant%20process. Accessed August 28, 2025.
-
5
National Cancer Institute, “Clinical Trials Frequently Asked Questions,” https://ccr.cancer.gov/clinical-trials/patients/faq. Accessed August 28, 2025.
-
6
ClinicalTrialBe, “How are side effects monitored in a clinical trial?”, https://clinicaltrial.be/en/posts/patients/how-are-side-effects-monitored-in-a-clinical-trial. Accessed August 28, 2025.
-
7
Clinical Trials Ontario, “Learning About Clinical Trials”, https://ctontario.ca/learn-about-trials/#:~:text=Now%20that%20I'm%20finished,related%20to%20this%20follow%20up. Accessed August 28, 2025.
-
8
Memorial Sloan Kettering Cancer Center, “Frequently Asked Questions (FAQs) About Cancer Clinical Trials, https://www.mskcc.org/cancer-care/clinical-trials/frequently-asked-questions. Accessed on August 28, 2025.
MRC-US-ALI-00060 09/25
